Coordinating brain-distributed network activities in memory resistant to extinction.
Certain persistent memories drive ill-advised actions. How such memories occur in the brain remains elusive. Here, by monitoring nerve cell activities across multiple brain regions in mice forming a memory for drug experience, we show that heightened cell coordination across brain regions underlies enduring memories. Toning down this enhanced activity coordination of distributed brain nerve cells allowed more appropriate behaviour to return.
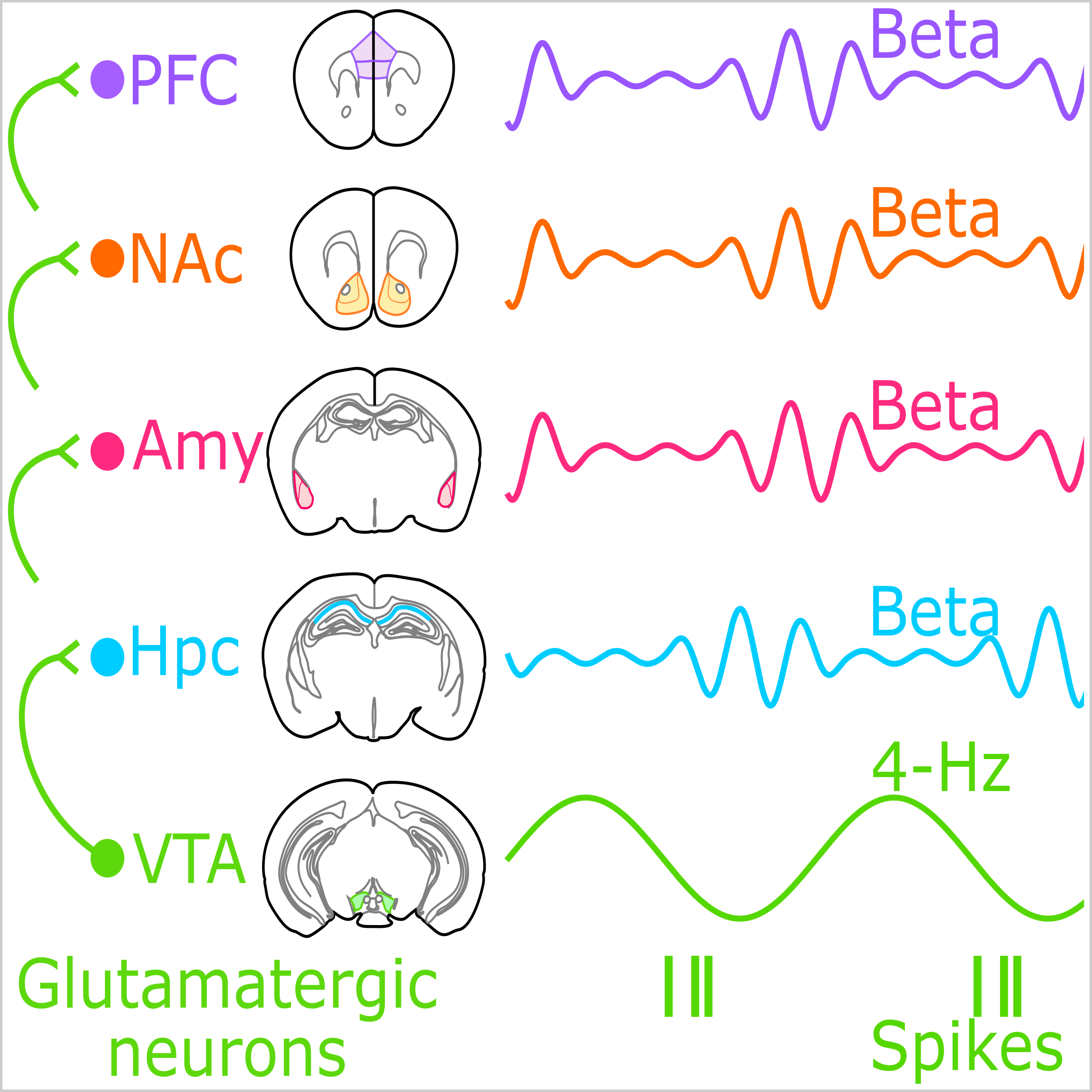
Certain memories resist extinction to continue invigorating maladaptive actions. The robustness of these memories could depend on their widely distributed implementation across populations of neurons in multiple brain regions. However, how dispersed neuronal activities are collectively organized to underpin a persistent memory-guided behavior remains unknown. To investigate this, we simultaneously monitored the prefrontal cortex, nucleus accumbens, amygdala, hippocampus, and ventral tegmental area (VTA) of the mouse brain from initial recall to post-extinction renewal of a memory involving cocaine experience. We uncover a higher-order pattern of short-lived beta-frequency (15-25 Hz) activities that are transiently coordinated across these networks during memory retrieval. The output of a divergent pathway from upstream VTA glutamatergic neurons, paced by a slower (4-Hz) oscillation, actuates this multi-network beta-band coactivation; its closed-loop phase-informed suppression prevents renewal of cocaine-biased behavior. Binding brain-distributed neural activities in this temporally structured manner may constitute an organizational principle of robust memory expression.
2019. Cell, 176(6):1393-1406.e16.
2016.Nat. Neurosci., 19(4):564-7.