A Hippocampus-Accumbens Tripartite Neuronal Motif Guides Appetitive Memory in Space.
Nerve cells in the brain cooperate to represent information and form memories. Yet, it is not clear how such internal representations of memory are ‘translated’ into behaviour. Here, we identify in the mouse a neural pathway linking two brain regions, the dorsal hippocampus and the nucleus accumbens, that is essential for turning memories of reward locations into goal-oriented actions.
Retrieving and acting on memories of food-predicting environments are fundamental processes for animal survival. Hippocampal pyramidal cells (PYRs) of the mammalian brain provide mnemonic representations of space. Yet the substrates by which these hippocampal representations support memory-guided behavior remain unknown. Here, we uncover a direct connection from dorsal CA1 (dCA1) hippocampus to nucleus accumbens (NAc) that enables the behavioral manifestation of place-reward memories. By monitoring neuronal ensembles in mouse dCA1→NAc pathway, combined with cell-type selective optogenetic manipulations of input-defined postsynaptic neurons, we show that dCA1 PYRs drive NAc medium spiny neurons and orchestrate their spiking activity using feedforward inhibition mediated by dCA1-connected parvalbumin-expressing fast-spiking interneurons. This tripartite cross-circuit motif supports spatial appetitive memory and associated NAc assemblies, being independent of dorsal subiculum and dispensable for both spatial novelty detection and reward seeking. Our findings demonstrate that the dCA1→NAc pathway instantiates a limbic-motor interface for neuronal representations of space to promote effective appetitive behavior.
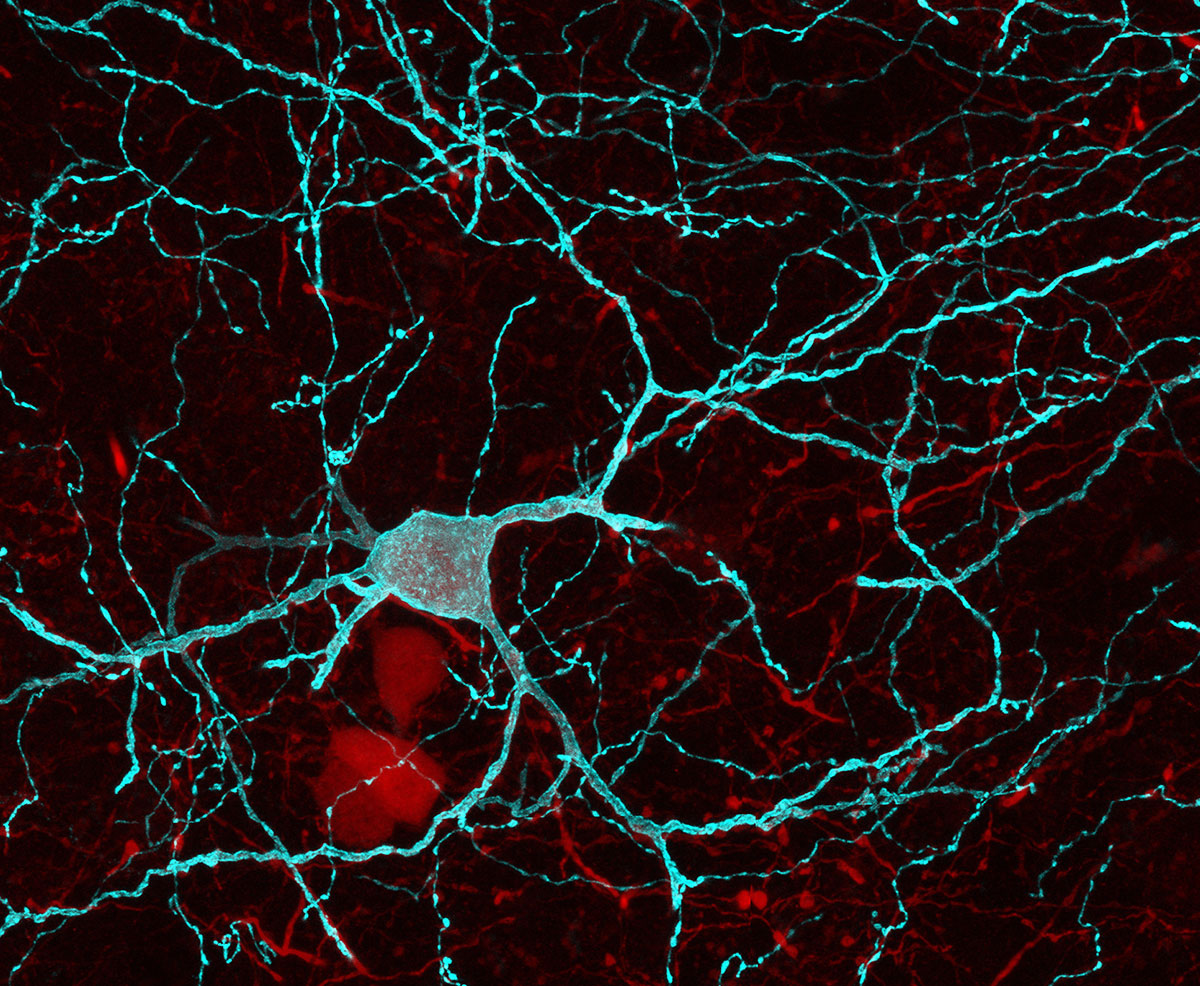
2019. Cell, 176(6):1393-1406.e16.
2024. Science, 385(6713):1120-1127.