Temporal evolution of beta bursts in the parkinsonian cortical and basal ganglia network.
Bursts of abnormal electrical rhythms occur in the brain of people with Parkinson’s disease. These bursts might slow movements. Here, we show that the timing of these oscillatory bursts at the brain surface is coupled to the activity of different populations of nerve cells in deep brain regions called the basal ganglia. The precise timing of the activity of these nerve cells could be used guide novel strategies for deep brain stimulation therapy.
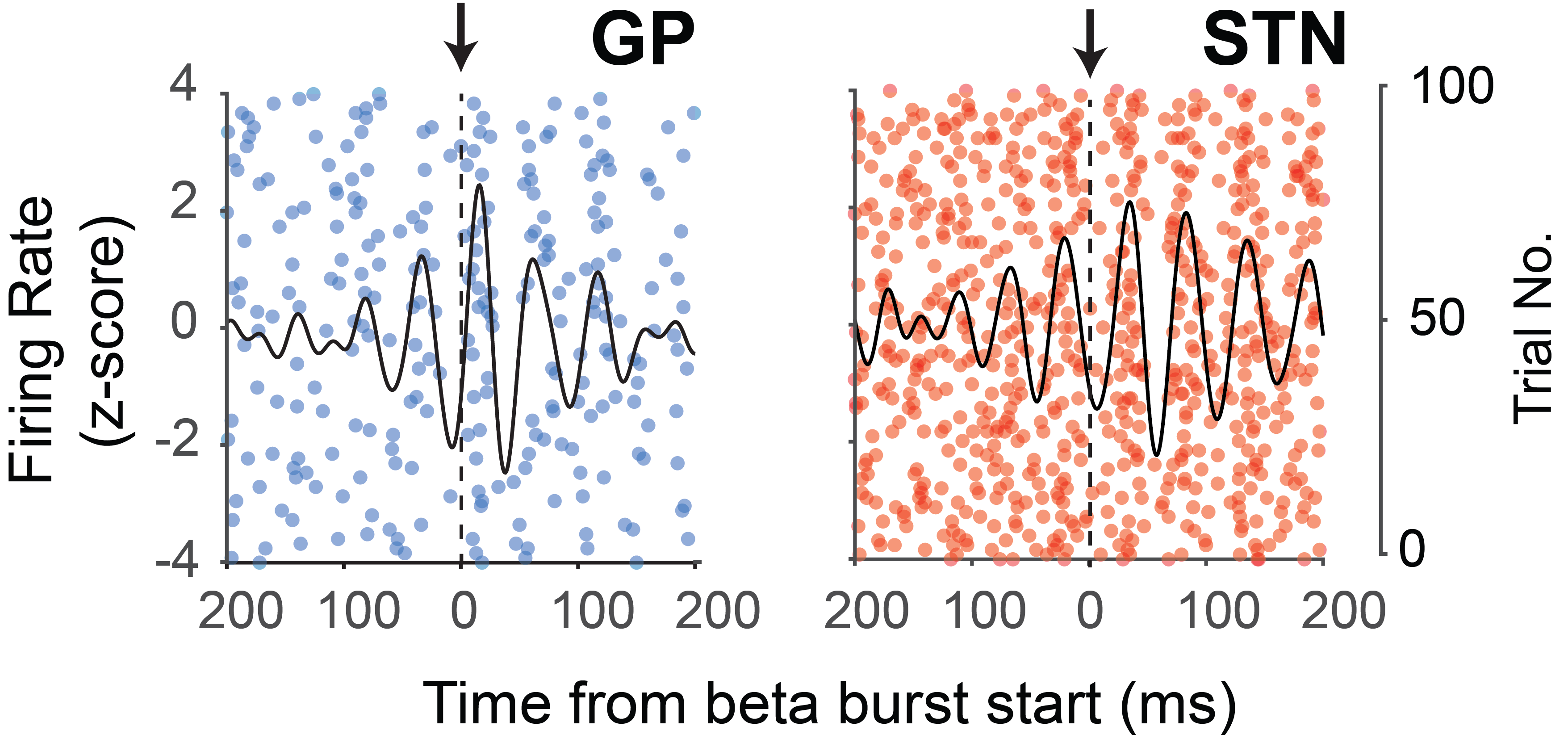
Beta frequency oscillations (15 to 35 Hz) in cortical and basal ganglia circuits become abnormally synchronized in Parkinson's disease (PD). How excessive beta oscillations emerge in these circuits is unclear. We addressed this issue by defining the firing properties of basal ganglia neurons around the emergence of cortical beta bursts (β bursts), transient (50 to 350 ms) increases in the beta amplitude of cortical signals. In PD patients, the phase locking of background spiking activity in the subthalamic nucleus (STN) to frontal electroencephalograms preceded the onset and followed the temporal profile of cortical β bursts, with conditions of synchronization consistent within and across bursts. Neuronal ensemble recordings in multiple basal ganglia structures of parkinsonian rats revealed that these dynamics were recapitulated in STN, but also in external globus pallidus and striatum. The onset of consistent phase-locking conditions was preceded by abrupt phase slips between cortical and basal ganglia ensemble signals. Single-unit recordings demonstrated that ensemble-level properties of synchronization were not underlain by changes in firing rate but, rather, by the timing of action potentials in relation to cortical oscillation phase. Notably, the preferred angle of phase-locked action potential firing in each basal ganglia structure was shifted during burst initiation, then maintained stable phase relations during the burst. Subthalamic, pallidal, and striatal neurons engaged and disengaged with cortical β bursts to different extents and timings. The temporal evolution of cortical and basal ganglia synchronization is cell type-selective, which could be key for the generation/ maintenance of excessive beta oscillations in parkinsonism.
2024. Neurobiol Dis, 201:106652.