Beta bursts in the parkinsonian cortico-basal ganglia network form spatially discrete ensembles.
People with Parkinson's disease have exaggerated rhythmic electrical activity at beta frequency across many brain areas. Understanding how these beta-oscillations synchronise neuronal activity is important for defining their role in the disease. Using recordings from many sites in the two of these areas, we show that different groups of neurons are synchronised separately by beta rhythms, rather all neurons being bound into the same activity.
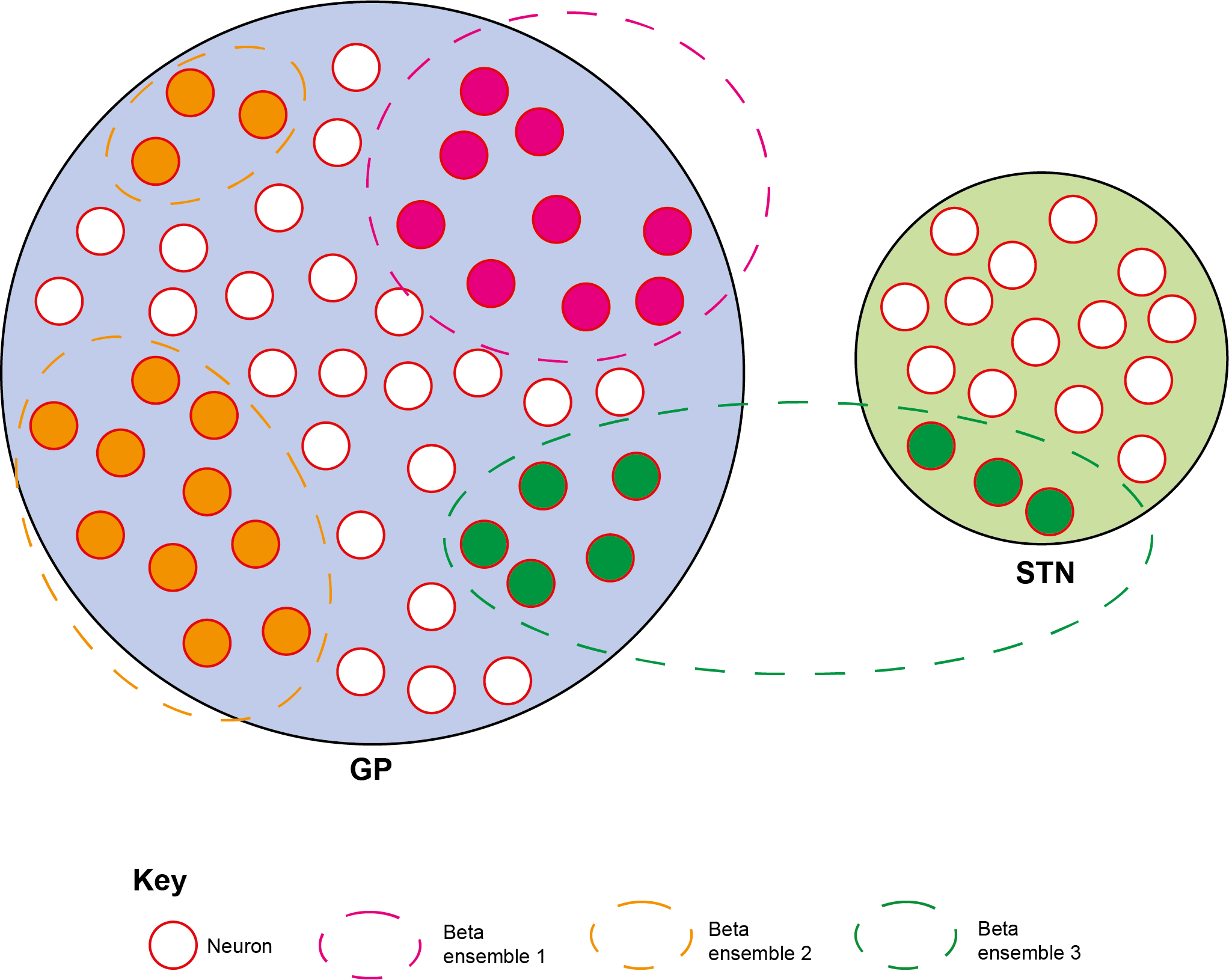
Defining spatial synchronisation of pathological beta oscillations is important, given that many theories linking them to parkinsonian symptoms propose a reduction in the dimensionality of the coding space within and/or across cortico-basal ganglia structures. Such spatial synchronisation could arise from a single process, with widespread entrainment of neurons to the same oscillation. Alternatively, the partially segregated structure of cortico-basal ganglia loops could provide a substrate for multiple ensembles that are independently synchronized at beta frequencies. Addressing this question requires an analytical approach that identifies groups of signals with a statistical tendency for beta synchronisation, which is unachievable using standard pairwise measures. Here, we utilized such an approach on multichannel recordings of background unit activity (BUA) in the external globus pallidus (GP) and subthalamic nucleus (STN) in parkinsonian rats. We employed an adapted version of a principle and independent component analysis-based method commonly used to define assemblies of single neurons (i.e., neurons that are synchronized over short timescales). This analysis enabled us to define whether changes in the power of beta oscillations in local ensembles of neurons (i.e., the BUA recorded from single contacts) consistently covaried over time, forming a "beta ensemble". Multiple beta ensembles were often present in single recordings and could span brain structures. Membership of a beta ensemble predicted significantly higher levels of short latency (<5 ms) synchrony in the raw BUA signal and phase synchronisation with cortical beta oscillations, suggesting that they comprised clusters of neurons that are functionally connected at multiple levels, despite sometimes being non-contiguous in space. Overall, these findings suggest that beta oscillations do not comprise of a single synchronisation process, but rather multiple independent activities that can bind both spatially contiguous and non-contiguous pools of neurons within and across structures. As previously proposed, such ensembles provide a substrate for beta oscillations to constrain the coding space of cortico-basal ganglia circuits.
2024. Neurobiol Dis, 201:106652.
2022. NPJ Parkinsons Dis, 8(1):88.