Hippocampal Offline Reactivation Consolidates Recently Formed Cell Assembly Patterns during Sharp Wave-Ripples.
Memories are believed to be represented in the brain by groups of nerve cells forming “cell assemblies”. Here, van de Ven and colleagues study a part of the brain called hippocampus and show that the preservation of cell assembly patterns representing a new spatial location depends on their ‘replay’ during sleep. This suggests that replay of brain activity is important for stabilizing memories.
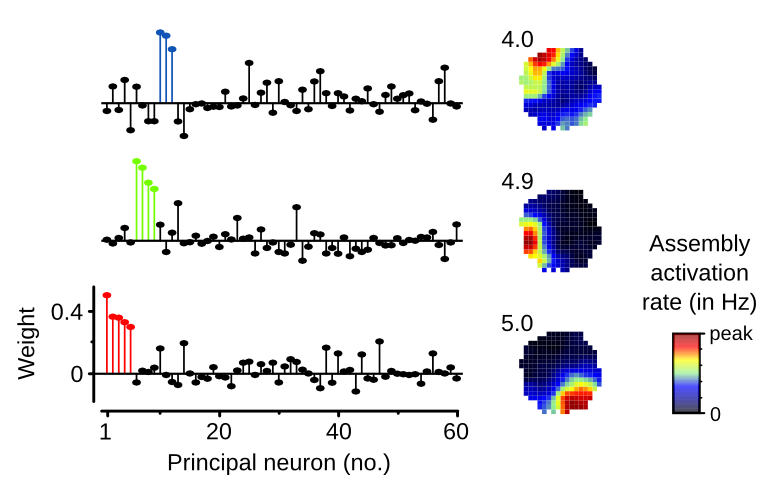
The ability to reinstate neuronal assemblies representing mnemonic information is thought to require their consolidation through offline reactivation during sleep/rest. To test this, we detected cell assembly patterns formed by repeated neuronal co-activations in the mouse hippocampus during exploration of spatial environments. We found that the reinstatement of assembly patterns representing a novel, but not a familiar, environment correlated with their offline reactivation and was impaired by closed-loop optogenetic disruption of sharp wave-ripple oscillations. Moreover, we discovered that reactivation was only required for the reinstatement of assembly patterns whose expression was gradually strengthened during encoding of a novel place. The context-dependent reinstatement of assembly patterns whose expression did not gain in strength beyond the first few minutes of spatial encoding was not dependent on reactivation. This demonstrates that the hippocampus can hold concurrent representations of space that markedly differ in their encoding dynamics and their dependence on offline reactivation for consolidation.
2024. Neuron, 112(22):3768-3781.e8.
2024. Science, 385(6713):1120-1127.
2021. Nat Neurosci, 24(3):326-330.

