The relative phases of basal ganglia activities dynamically shape effective connectivity in Parkinson's disease.
Scientists believe that brain waves in different regions have to fall into register to allow information flow between them to have maximal effect. We show that the falling out of register is tightly controlled in brain motor circuits by dopamine, a key chemical messenger lost in Parkinson’s. Without dopamine, information channels jam open, and dynamic control of information flow is compromised.
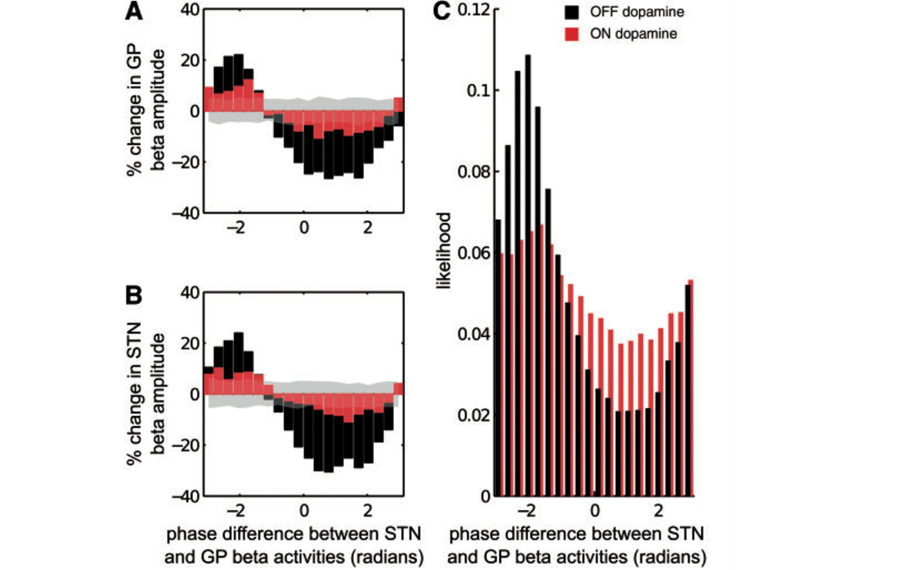
Optimal phase alignment between oscillatory neural circuits is hypothesized to optimize information flow and enhance system performance. This theory is known as communication-through-coherence. The basal ganglia motor circuit exhibits exaggerated oscillatory and coherent activity patterns in Parkinson's disease. Such activity patterns are linked to compromised motor system performance as evinced by bradykinesia, rigidity and tremor, suggesting that network function might actually deteriorate once a certain level of net synchrony is exceeded in the motor circuit. Here, we characterize the processes underscoring excessive synchronization and its termination. To this end, we analysed local field potential recordings from the subthalamic nucleus and globus pallidus of five patients with Parkinson's disease (four male and one female, aged 37-64 years). We observed that certain phase alignments between subthalamic nucleus and globus pallidus amplified local neural synchrony in the beta frequency band while others either suppressed it or did not induce any significant change with respect to surrogates. The increase in local beta synchrony directly correlated with how long the two nuclei locked to beta-amplifying phase alignments. Crucially, administration of the dopamine prodrug, levodopa, reduced the frequency and duration of periods during which subthalamic and pallidal populations were phase-locked to beta-amplifying alignments. Conversely ON dopamine, the total duration over which subthalamic and pallidal populations were aligned to phases that left beta-amplitude unchanged with respect to surrogates increased. Thus dopaminergic input shifted circuit dynamics from persistent periods of locking to amplifying phase alignments, associated with compromised motoric function, to more dynamic phase alignment and improved motoric function. This effect of dopamine on local circuit resonance suggests means by which novel electrical interventions might prevent resonance-related pathological circuit interactions.
2021. Brain, 144(3):781-788.
2016.PLoS Comput. Biol., 12(9):e1005062.