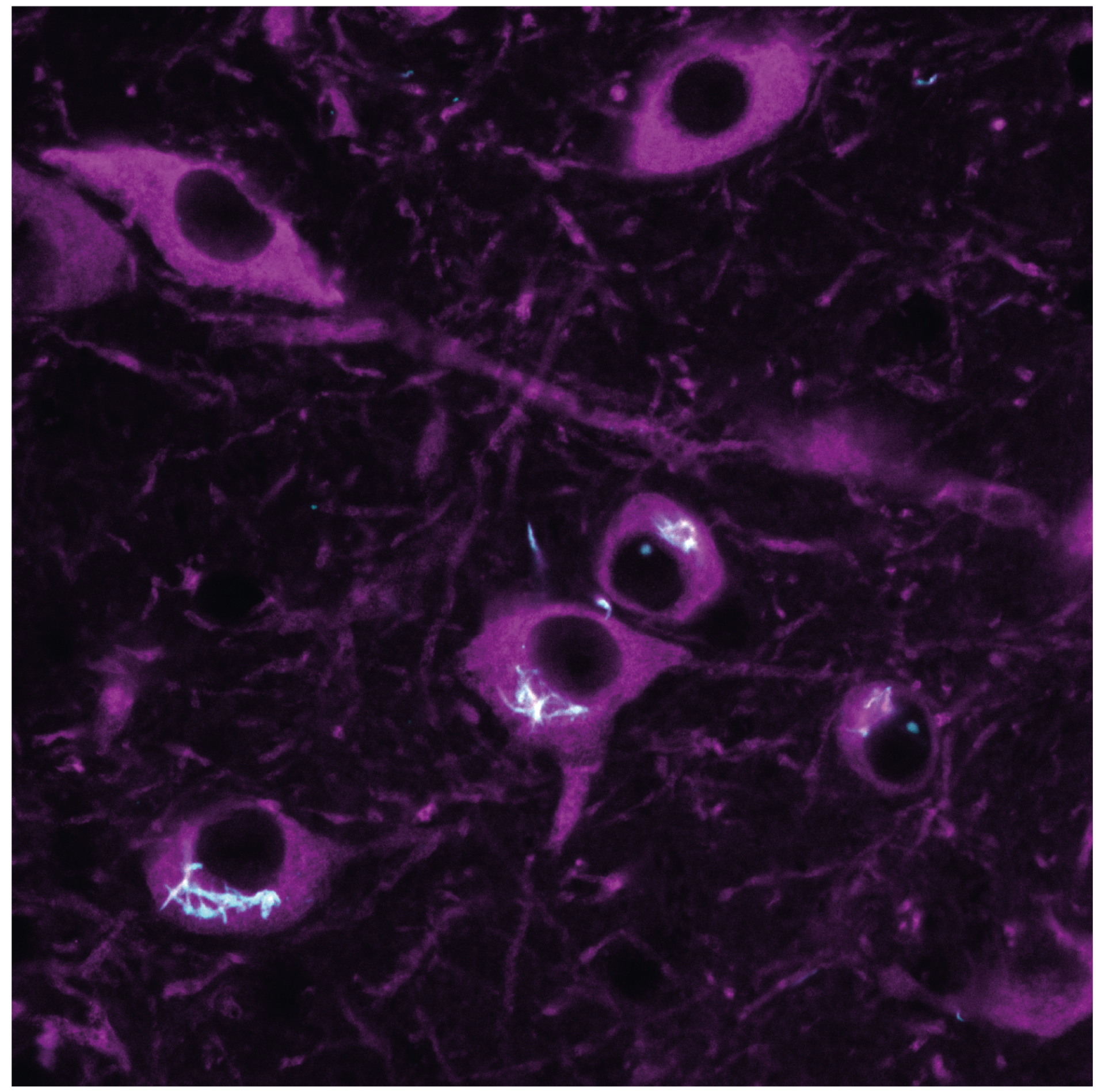
Large-scale visualization of α-synuclein oligomers in Parkinson's disease brain tissue.
In Parkinson’s, vulnerable nerve cells often contain clumps of a protein called α-synuclein. Here, we worked with colleagues from across the UK to develop a new method to see tiny clumps of α-synuclein in brain tissue from human and mouse models. The new method showed that clumps become larger and more numerous as people develop Parkinson’s, giving important clues about why and when cells die in the disease.
Parkinson's disease (PD) is a neurodegenerative condition characterized by the presence of intraneuronal aggregates containing fibrillar ɑ-synuclein known as Lewy bodies. These large end-stage species are formed by smaller soluble protein nanoscale assemblies, often termed oligomers, which are proposed as early drivers of pathogenesis. Until now, this hypothesis has remained controversial, at least in part because it has not been possible to directly visualize nanoscale assemblies in human brain tissue. Here we present Advanced Sensing of Aggregates-Parkinson's Disease, an imaging method to generate large-scale α-synuclein aggregate maps in post-mortem human brain tissue. We combined autofluorescence suppression with single-molecule fluorescence microscopy, which together enable the detection of nanoscale α-synuclein aggregates. To demonstrate the use of this platform, we analysed ~1.2 million nanoscale aggregates from the anterior cingulate cortex in human post-mortem brain samples from patients with PD and healthy controls. Our data reveal a disease-specific shift in a subpopulation of nanoscale assemblies that represent an early feature of the proteinopathy that underlies PD. We anticipate that quantitative information about this distribution provided by Advanced Sensing of Aggregates-Parkinson's Disease will enable mechanistic studies to reveal the pathological processes caused by α-synuclein aggregation.
2025. Nat Biomed Eng (e-Pub ahead of print).
2021. Nat Commun, 12(1):5185.
