Stagg Group
Our group aims to define the physiological changes that happen in the human brain during the learning of new motor skills in health and in a range of neurological conditions. To do this, we use a powerful combination of techniques for assessing and influencing brain activity, including neuroimaging and non-invasive brain stimulation. We hope that by increasing the understanding of how the brain responds to learning new tasks, we can design new therapies that have the potential to improve functional recovery after stroke.
There is a significant clinical need to improve functional recovery after stroke. New and imporved therapies are urgently required. However, the neurophysiological changes that underlie behavioural improvements are incompletely understood, which presents a major challenge for developing novel adjunct therapies.
The overarching goal of our group is therefore to understand the neural changes underpinning the acquisition of motor skills, both in health and after brain injury such as a stroke, with the ultimate aim of developing novel therapies to optimise functional recovery after stroke. Behaviourally-relevant neuroplasticity occurs across a range of spatial scales, and we therefore use multimodal neuroimaging approaches to capture this spatial diversity.
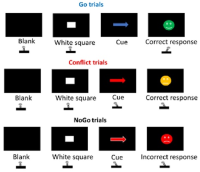
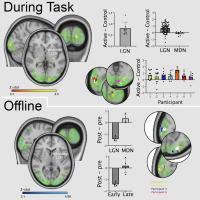
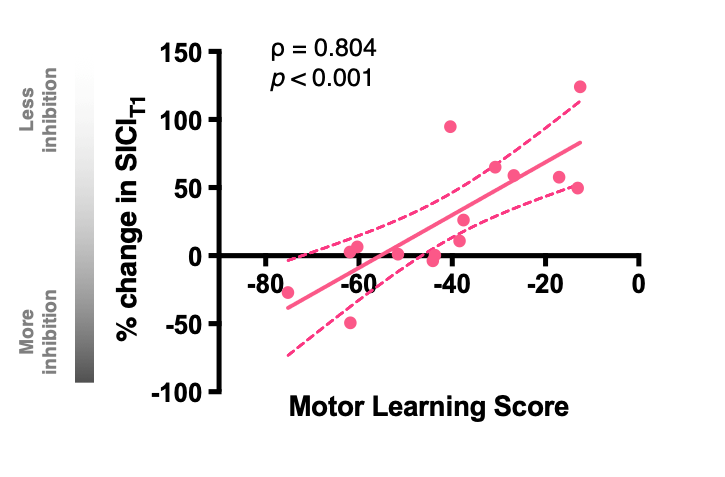
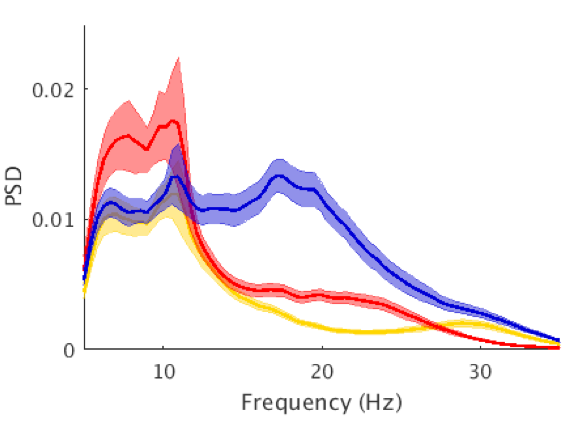
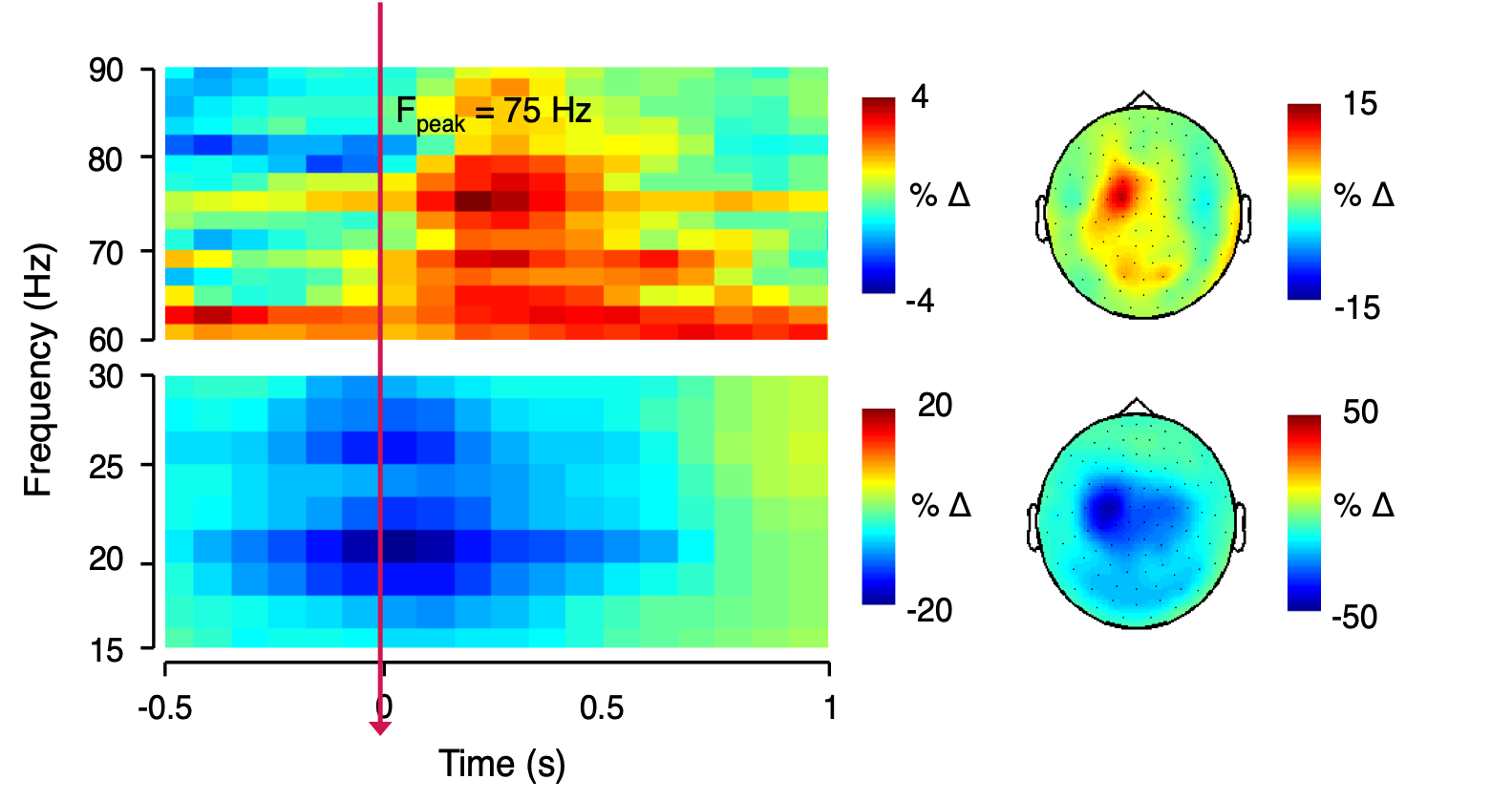
We focus particularly on the primary motor cortex (M1) and use novel Magnetic Resonance Spectroscopy (MRS) and Magnetic Resonance Imaging (MRI) to quantify local GABAergic concentrations. Our work studies the fundamental relationship between local inhibitory activity and dynamic activity as revealed using electrophysiological approaches, and to network-level connectivity as determined by MRI. In combination with these imaging approaches. we use non-invasive brain stimulation approaches to manipulate on-going activity to determine causality of the identified relationships.
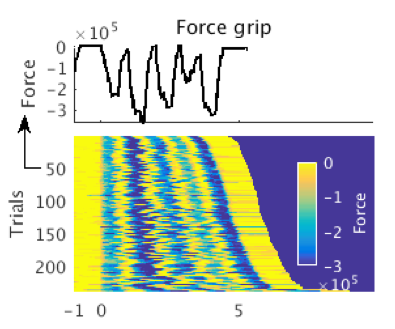
In parallel with our human work, we are beginning to use rodent models to investigate the same neural signals we see in the human. These animal models afford the opportunity to map the contributions of specific cell types with a precision this is not yet possible in the clinic.
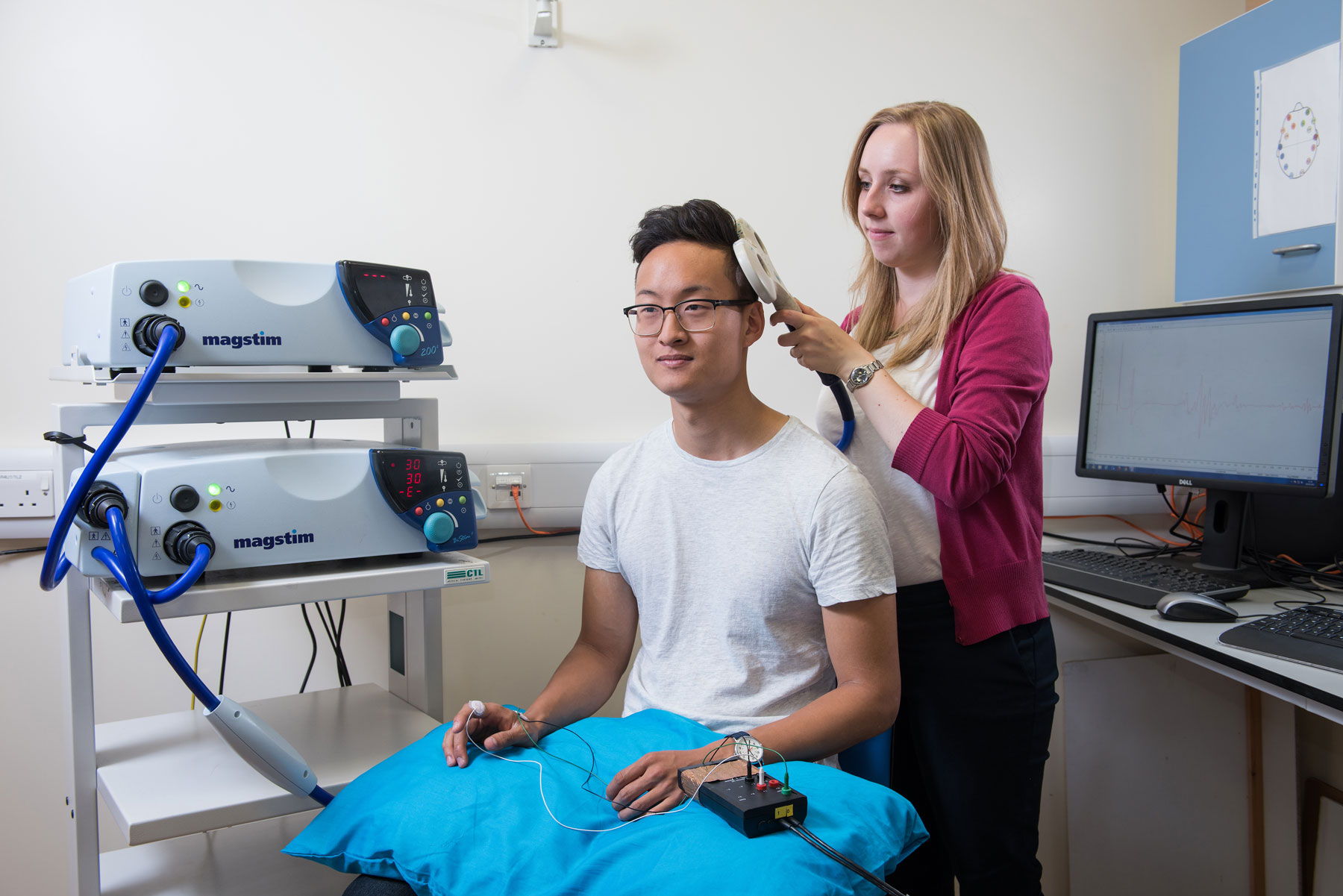
We use the basic neuroscientific findings arising from both the human and rodent work to design novel transcranial stimulation approaches to specifically modulate on-going brain activity in order to maximise learning by driving underlying plasticity. We utilise transcranial electrical stimulation approaches using novel waveforms as well as developing new approaches such as transcranial ultrasound for neuromodulation. We then test the efficacy of these approaches in Phase II clinical trials.
Taken together, our work spans basic and translational neuroscience, utilising appropriate methods to gain a complete understanding of this complex, important and currently under-met clinical need.
- Interplay between local inhibition and dynamic activity in primary motor cortex.
- Defining the role of local inhibition in modulating connectivity across brain networks.
- Modulating cortical excitability to optimise acquisition of motor skills.
- Driving dynamic brain activity to modulate neuroplasticity.
- Developing novel adjunct therapies for functional recovery after stroke.
Currently, our ability to improve functional outcome after stroke is limited both by a paucity of understanding and a lack of anatomical specificity in stimulation approaches. Ultimately, we hope to develop individualised approaches, with the ability to target specific activity with a far greater precision that we currently have, to maximise outcome for every patient. Developing novel stimulation approaches, such as ultrasound, and refining those we already have, are the next steps towards achieving this long-term goal.
- Magnetic Resonance Imaging and Spectroscopy.
- Magnetoencephalography.
- Non-invasive brain stimulation.
- Rodent in vivo electrophysiology.
- Quantification of voluntary behaviours.
We are committed to fostering an inclusive work environment that celebrates diversity and promotes equal opportunity within our group and the wider MRC BNDU.

The enthusiastic BNDU team of scientists. (L-R) Shenghong, Chiara, Rosie and Ioana.

Mary Muers (far right) moderates an interactive session about training and career development opportunities.
Recent Preprints