Subthalamic nucleus activity dynamics and limb movement prediction in Parkinson's disease.
In Parkinson’s disease, brief episodes (or ‘bursts’) of a specific pattern of brain waves are associated with difficulty in moving. Here, we show that these bursts restrict the ability of a nerve cell circuit in the brain to signal relevant information about intended movements.
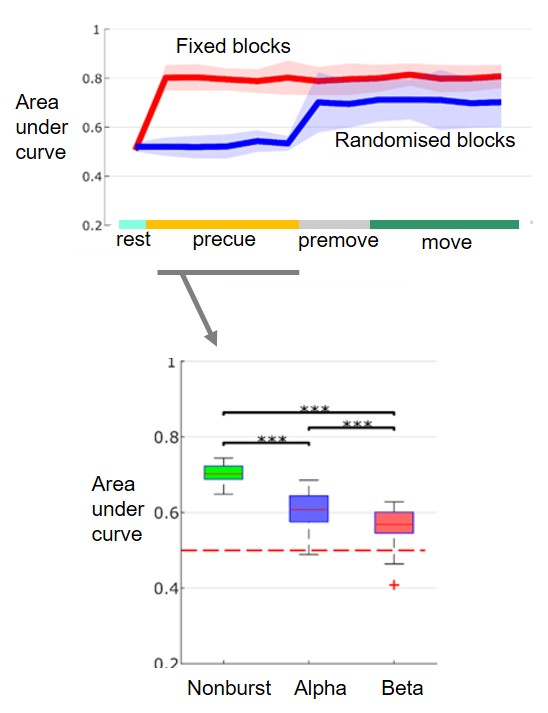
Whilst exaggerated bursts of beta frequency band oscillatory synchronization in the subthalamic nucleus have been associated with motor impairment in Parkinson's disease, a plausible mechanism linking the two phenomena has been lacking. Here we test the hypothesis that increased synchronization denoted by beta bursting might compromise information coding capacity in basal ganglia networks. To this end we recorded local field potential activity in the subthalamic nucleus of 18 patients with Parkinson's disease as they executed cued upper and lower limb movements. We used the accuracy of local field potential-based classification of the limb to be moved on each trial as an index of the information held by the system with respect to intended action. Machine learning using the naïve Bayes conditional probability model was used for classification. Local field potential dynamics allowed accurate prediction of intended movements well ahead of their execution, with an area under the receiver operator characteristic curve of 0.80 ± 0.04 before imperative cues when the demanded action was known ahead of time. The presence of bursts of local field potential activity in the alpha, and even more so, in the beta frequency band significantly compromised the prediction of the limb to be moved. We conclude that low frequency bursts, particularly those in the beta band, restrict the capacity of the basal ganglia system to encode physiologically relevant information about intended actions. The current findings are also important as they suggest that local subthalamic activity may potentially be decoded to enable effector selection, in addition to force control in restorative brain-machine interface applications.
2020. J. Neurosci., 40(7):1571-1580.