Impaired striatal glutathione-ascorbate metabolism induces transient dopamine increase and motor dysfunction.
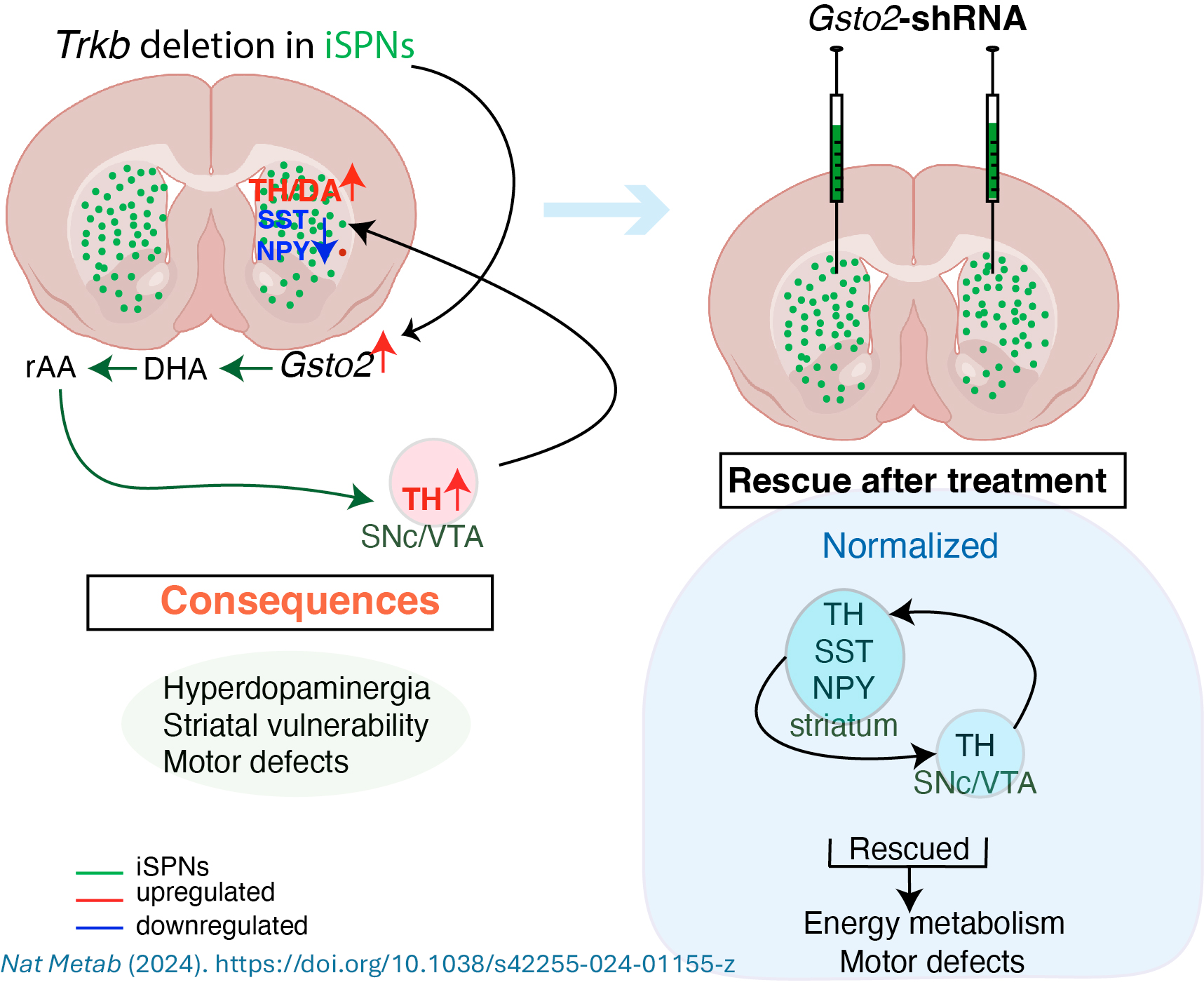
Some types of nerve cell are more vulnerable than others in neurodegenerative brain diseases like Huntington’s. Here, we used mouse models to show that loss of a gene called Trkb in the nerve cell type most affected in Huntington’s increased expression of an enzyme called GSTO2. This led to dopamine signalling changes, impaired energy metabolism, progressive degeneration and abnormal movement. Suppression of GSTO2 corrected some of these problems.
Identifying initial triggering events in neurodegenerative disorders is critical to developing preventive therapies. In Huntington's disease (HD), hyperdopaminergia-probably triggered by the dysfunction of the most affected neurons, indirect pathway spiny projection neurons (iSPNs)-is believed to induce hyperkinesia, an early stage HD symptom. However, how this change arises and contributes to HD pathogenesis is unclear. Here, we demonstrate that genetic disruption of iSPNs function by Ntrk2/Trkb deletion in mice results in increased striatal dopamine and midbrain dopaminergic neurons, preceding hyperkinetic dysfunction. Transcriptomic analysis of iSPNs at the pre-symptomatic stage showed de-regulation of metabolic pathways, including upregulation of Gsto2, encoding glutathione S-transferase omega-2 (GSTO2). Selectively reducing Gsto2 in iSPNs in vivo effectively prevented dopaminergic dysfunction and halted the onset and progression of hyperkinetic symptoms. This study uncovers a functional link between altered iSPN BDNF-TrkB signalling, glutathione-ascorbate metabolism and hyperdopaminergic state, underscoring the vital role of GSTO2 in maintaining dopamine balance.
2024. Nat Metab, 6(11):2100-2117.
2016.Cereb. Cortex, 26(10):3977-90.