Behavior-Dependent Activity and Synaptic Organization of Septo-hippocampal GABAergic Neurons Selectively Targeting the Hippocampal CA3 Area.
The rhythmic electrical activity of brain nerve cells supports learning and behaviour. Here, we identify and characterize Teevra cells as the most rhythmic cells in the medial septum, a deep-lying brain area that sends connections up to the hippocampus, a brain region important for memory. Teevra cells could play key roles in coordinating the electrical activity of nerve cells in the hippocampus.
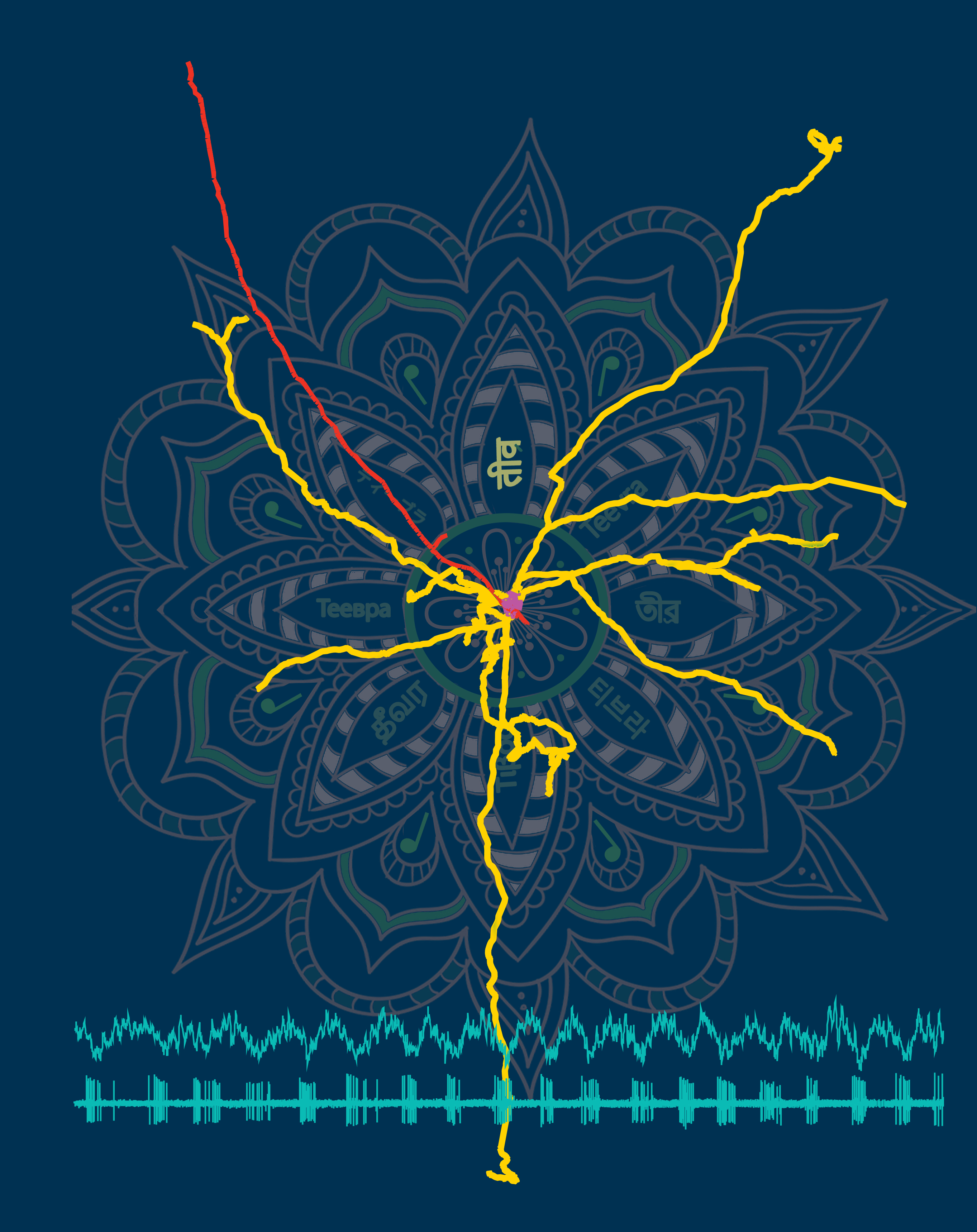
Rhythmic medial septal (MS) GABAergic input coordinates cortical theta oscillations. However, the rules of innervation of cortical cells and regions by diverse septal neurons are unknown. We report a specialized population of septal GABAergic neurons, the Teevra cells, selectively innervating the hippocampal CA3 area bypassing CA1, CA2, and the dentate gyrus. Parvalbumin-immunopositive Teevra cells show the highest rhythmicity among MS neurons and fire with short burst duration (median, 38 ms) preferentially at the trough of both CA1 theta and slow irregular oscillations, coincident with highest hippocampal excitability. Teevra cells synaptically target GABAergic axo-axonic and some CCK interneurons in restricted septo-temporal CA3 segments. The rhythmicity of their firing decreases from septal to temporal termination of individual axons. We hypothesize that Teevra neurons coordinate oscillatory activity across the septo-temporal axis, phasing the firing of specific CA3 interneurons, thereby contributing to the selection of pyramidal cell assemblies at the theta trough via disinhibition.