Structural and molecular heterogeneity of calretinin-expressing interneurons in the rodent and primate striatum.
The striatum is a brain area important for movement. Nerve cells called interneurons help control communication within striatum. We investigated the structure, molecular architecture and electrical activity of interneurons that make a protein called calretinin. We define three different types of calretinin interneuron that could play complementary roles in controlling other cells in striatum.
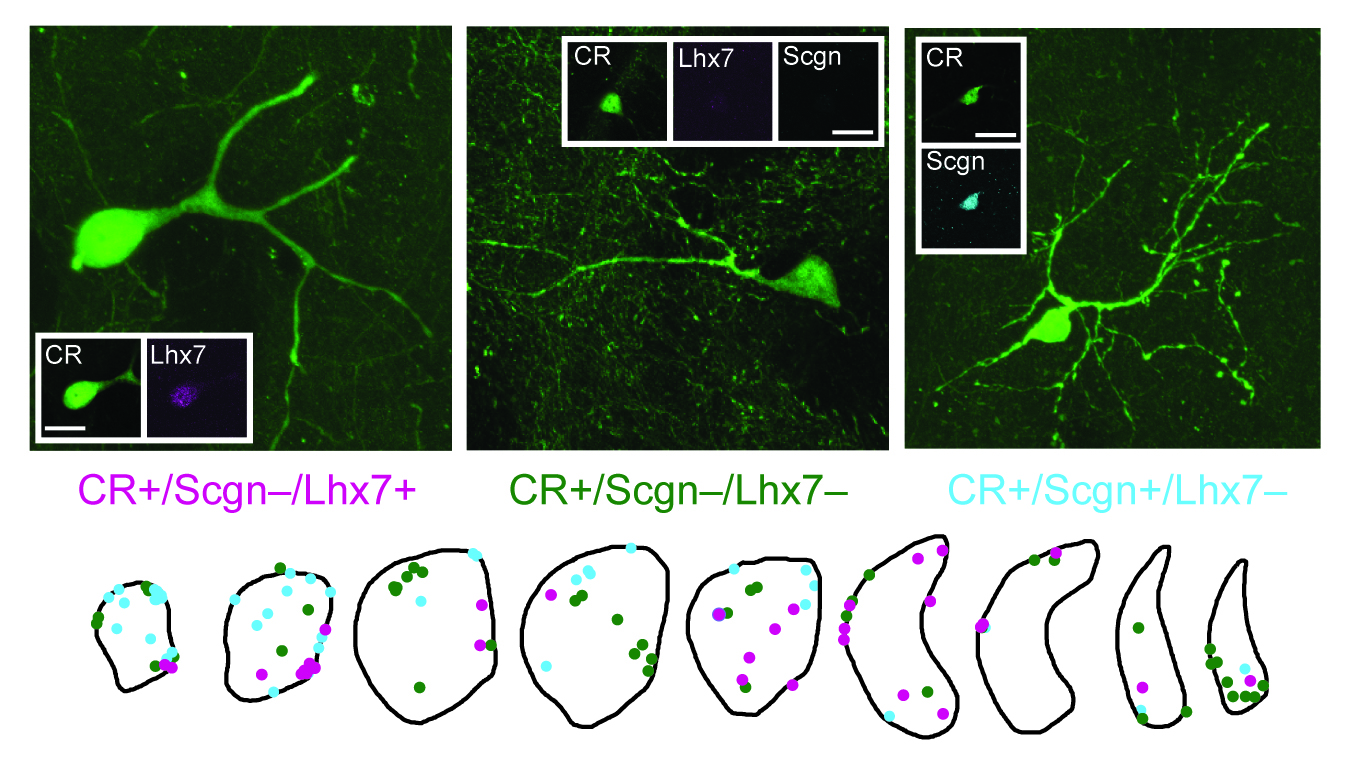
Calretinin-expressing (CR+) interneurons are the most common type of striatal interneuron in primates. However, because CR+ interneurons are relatively scarce in rodent striatum, little is known about their molecular and other properties, and they are typically excluded from models of striatal circuitry. Moreover, CR+ interneurons are often treated in models as a single homogenous population, despite previous descriptions of their heterogeneous structures and spatial distributions in rodents and primates. Here, we demonstrate that, in rodents, the combinatorial expression of secretagogin (Scgn), specificity protein 8 (SP8) and/or LIM homeobox protein 7 (Lhx7) separates striatal CR+ interneurons into three structurally and topographically distinct cell populations. The CR+/Scgn+/SP8+/Lhx7- interneurons are small-sized (typically 7-11 µm in somatic diameter), possess tortuous, partially spiny dendrites, and are rostrally biased in their positioning within striatum. The CR+/Scgn-/SP8-/Lhx7- interneurons are medium-sized (typically 12-15 µm), have bipolar dendrites, and are homogenously distributed throughout striatum. The CR+/Scgn-/SP8-/Lhx7+ interneurons are relatively large-sized (typically 12-20 µm), and have thick, infrequently branching dendrites. Furthermore, we provide the first in vivo electrophysiological recordings of identified CR+ interneurons, all of which were the CR+/Scgn-/SP8-/Lhx7- cell type. In the primate striatum, Scgn co-expression also identified a topographically distinct CR+ interneuron population with a rostral bias similar to that seen in both rats and mice. Taken together, these results suggest that striatal CR+ interneurons comprise at least three molecularly, structurally, and topographically distinct cell populations in rodents. These properties are partially conserved in primates, in which the relative abundance of CR+ interneurons suggests that they play a critical role in striatal microcircuits.
2023. eNeuro, 10(7).