Beta-triggered adaptive deep brain stimulation during reaching movement in Parkinson's disease.
In people with Parkinson’s disease, we compared adaptive deep brain stimulation (DBS) to continuous DBS. The adaptive DBS was triggered by brain wave activity at frequencies called ‘beta’, detected in the subthalamic nucleus region. The two types of DBS were equally effective at improving reaching arm movements, while adaptive DBS was less disruptive of higher-frequency waves, called 'gamma', occurring during these movements.
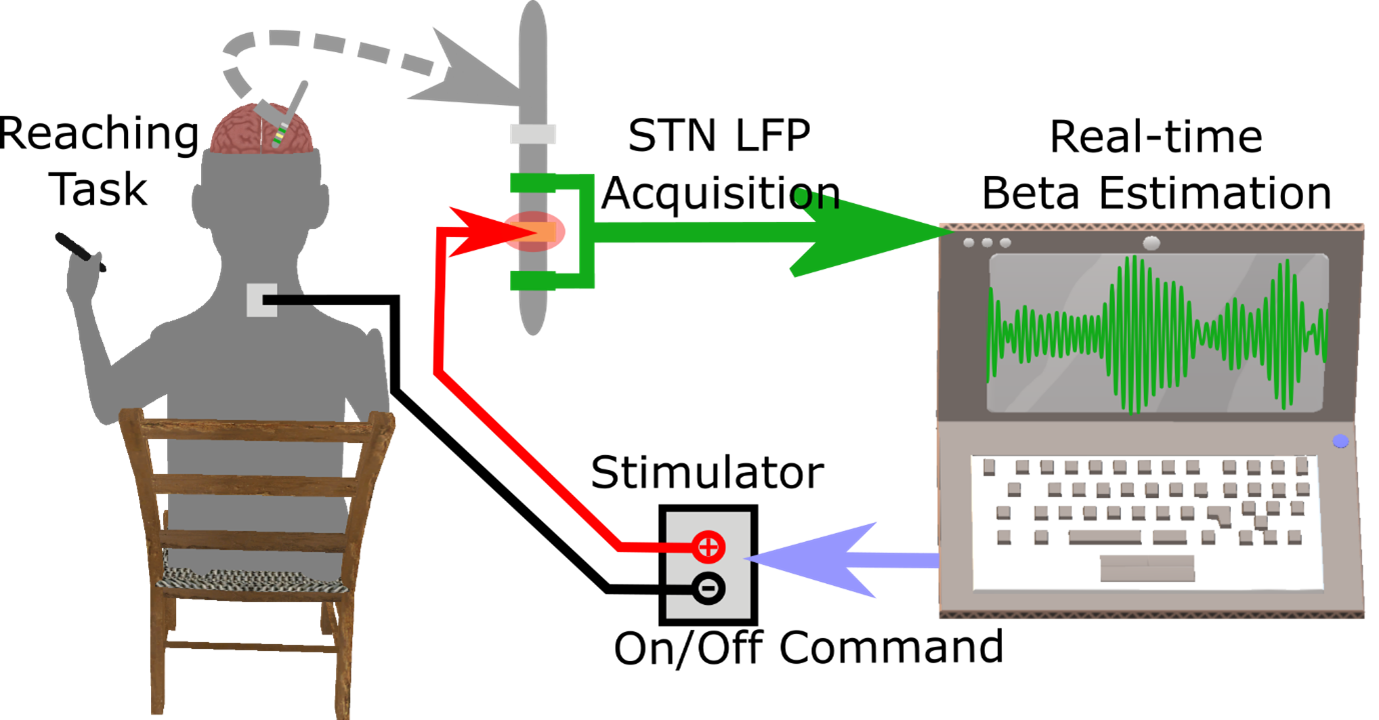
Subthalamic nucleus (STN) beta-triggered adaptive deep brain stimulation (ADBS) has been shown to provide clinical improvement comparable to conventional continuous DBS (CDBS) with less energy delivered to the brain and less stimulation induced side effects. However, several questions remain unanswered. First, there is a normal physiological reduction of STN beta band power just prior to and during voluntary movement. ADBS systems will therefore reduce or cease stimulation during movement in people with Parkinson's disease and could therefore compromise motor performance compared to CDBS. Second, beta power was smoothed and estimated over a time period of 400 ms in most previous ADBS studies, but a shorter smoothing period could have the advantage of being more sensitive to changes in beta power, which could enhance motor performance. In this study, we addressed these two questions by evaluating the effectiveness of STN beta-triggered ADBS using a standard 400 ms and a shorter 200 ms smoothing window during reaching movements. Results from 13 people with Parkinson's disease showed that reducing the smoothing window for quantifying beta did lead to shortened beta burst durations by increasing the number of beta bursts shorter than 200 ms and more frequent switching on/off of the stimulator but had no behavioural effects. Both ADBS and CDBS improved motor performance to an equivalent extent compared to no DBS. Secondary analysis revealed that there were independent effects of a decrease in beta power and an increase in gamma power in predicting faster movement speed, while a decrease in beta event related desynchronization (ERD) predicted quicker movement initiation. CDBS suppressed both beta and gamma more than ADBS, whereas beta ERD was reduced to a similar level during CDBS and ADBS compared with no DBS, which together explained the achieved similar performance improvement in reaching movements during CDBS and ADBS. In addition, ADBS significantly improved tremor compared with no DBS but was not as effective as CDBS. These results suggest that STN beta-triggered ADBS is effective in improving motor performance during reaching movements in people with Parkinson's disease, and that shortening of the smoothing window does not result in any additional behavioural benefit. When developing ADBS systems for Parkinson's disease, it might not be necessary to track very fast beta dynamics; combining beta, gamma, and information from motor decoding might be more beneficial with additional biomarkers needed for optimal treatment of tremor.
2023. Brain, 146(12):5015-5030.
2023. Mov Disord, 38(5):818-830.